Generation of the electrophile some more details
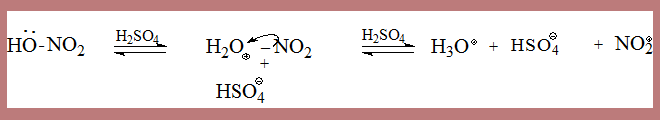
The electrophile Nironium ion is generated as shown.
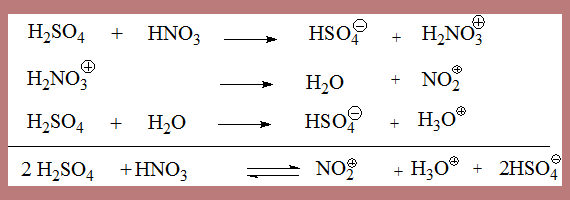
Nitric acid gets protonated, in a medium such as sulphuric acid to form H2O+.NO2 which looses water to form nitronium ion. The mixture thus has four ions.
The function of sulphuric acid is not as a reagent for absorbing the water that is formed
Solution of nitric acid in pure sulphuric acid shows a fourfold depression in freezing point which can be interpreted as due to the presence of four ions.
Nitration is very slow in the absence of sulphuric acid. Sulphuric acid itself has no influence on benzene under the conditions of the reaction. So sulphuric acid is acting on nitric acid rather than on benzene.
The electrophile in the reaction is NO2+, why not H3O+
Though Oxygen atom in H3O+ has a positive charge it is still not an electrophilic centre, because the atom has a complete octet in its outermost shell hence cannot accommodate any more electrons.
Nitronium ion as an electrophile
The presence of NO2+ in solution and as part of a salt has been confirmed.
Nitronium perchlorate (NO2+ClO4-), Nitronium fluoroborate (NO2+BF4-), have been prepared and are found to be effective nitrating agents at even sub zero temperatures.
A line in the Raman spectrum at 1400 cm-1, corresponds to a linear triatomic species.
The function of sulphuric acid is to just generate the nitronium ion by interacting with nitric acid, if that is so any other strong acid should promote nitration. It has been found to be so, HClO4- and HF + BF3 are also effective.
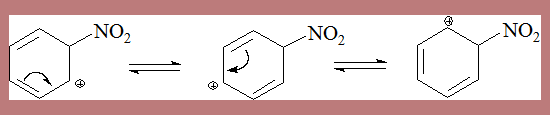
The actual isolation of σ complex in some processes lends credibility to the mechanism as in the following case
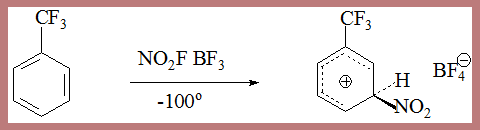
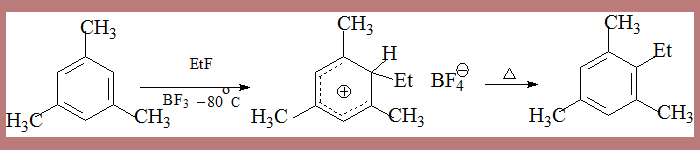
In the second step HSO4- depotonates the σ complex leading to nitrobenzene. The σ complex is unstable, by loss of a proton it can form a very stable aromatic species. This happens when a base from the medium is available.
Nitration is not a single step process.
Evidence:
Nitration is carried out using C6H6 and C6D6under identical conditions and the rate is measured in each case (kH , kD). If the C-H or C-D bond is breaking in the rate determining step the two rate constants should be different kD should be much higher than kH, ( about 7). In actual fact they have been found to be almost similar in value. Therefore the C-H or C-D bond is not breaking in the rate determining step, that is it is breaking in another step. Conclusion is that the mechanism is not a single step process.
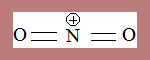
Structure of Nitronium ion
The species is linear-bond angle: 180o . Distance between Nitrogen and oxygen : 115pm
Nitrogen atom is sp hybridised.
The sigma complex is resonance stabilised.
In the generation of electrophile four ions are involved, how do you know?
The combination of sulphuric and nitric acids is called the nitration mixture. Generally it is in the ratio .....